Recurrent Pregnancy Loss (Abortions); Thalassemia, Duchenne muscular dystrophy (DMD), Sickle Cell Anaemia
Recurring Pregnancy Loss (RPL)
Recurrent miscarriage - ≥ 3 pregnancy loss; affects about 1% of all women.
It is a heterogeneous condition with multifactorial aetiology
Advanced Maternal age and previous miscarriages are two independent risk factors for a further miscarriage.
1. Genetic factor:
3–5% of couples with RPL; anyone may be a balanced chromosomal translocation carrier; 5–10% chance of a pregnancy with an unbalanced translocation in subsequent pregnancy.
2. Anatomical factors
Uterine anomalies in RPL is in range of 2% and 8%.
3. Cervical weaknesses
Mid-trimester miscarriage; history of mid trimester miscarriage with spontaneous rupture of membranes and painless cervical dilatation.
4. Endocrine factors
Routine screening for occult diabetes and thyroid disease may be helpful.
Polycystic ovarian syndrome (PCOS) has been linked to miscarriage; significantly common among women with recurrent miscarriage (41%) when compared with the general population (22%).
5. Immune factors
Antiphospholipid syndrome (APS): Adverse pregnancy outcomes include (a) ≥ 3 consecutive miscarriages < 10 weeks of gestation, (b) ≥ 1 normal foetal deaths > 10 week of gestation (c) ≥ 1 preterm births ; < 34 week of gestation due to severe pre-eclampsia, eclampsia or placental insufficiency.
APS with chronic inflammatory disorders; Systemic lupus erythematosus
It inhibits trophoblastic function, differentiation, might lead to thrombosis of the uteroplacental vasculature.
Antiphospholipid antibodies are present in 15% of women with RPL as compared to < 2% of controls. In women with RPL associated with aPL, the live birth rate of pregnancies with no pharmacological intervention is in the range of 10%.
Live birth rate with RPL - 40% with aspirin only; significantly increases to 70% with aspirin & low molecular weight heparin (LMWH). Aspirin & LMWH significantly reduces the pregnancy losses by 50%.
Alloimmune factors
Immunotherapy (paternal cell immunisation), third-party donor leucocytes and intravenous immunoglobulin (IVIG), in women with RPL does not improve the live birth rate.
Infective agents
TORCH screening
6. Inherited thrombophilic defects
Factor V Leiden mutation; deficiencies of protein C/S and antithrombin III, hyperhomocysteinaemia and prothrombin gene mutation are established factors for thrombosis of uteroplacental circulation.
7. Unexplained recurrent miscarriage
A significant proportion of cases of recurrent miscarriage remain unexplained, despite detailed investigation. The prognosis for a successful future pregnancy with supportive care alone is in the region of 75%.
Thalassemia
One of the commonest genetic disease
1 in 550-600 induvial is beta thalassemia carrier around 250 million people (4.5% of world population) is affected by Thalassemia.
Thalassemia Major - Requires life-long repeated blood transfusions, on monthly basis.
Thalassemia can be corrected by Bone Marrow Transplantation.
If both parents are thalassemia carrier; there is 25% (1 in 4) chance to have thalassemia major foetus
Prenatal testing
Chorionic villus sampling (CVS)
Duchenne muscular dystrophy (DMD)
X-linked recessive; affects around 1 in 3,600 boys
Mutation in dystrophin gene; located on X chromosome.
Males are affected while the females are usually carriers and asymptomatic.
Genetic testing
To identify affected male & Carrier (Female partner)
Sickle Cell Anaemia
Sickle-cell anaemia; also known as “HbSS” disease.
Autosomal recessive in nature; both partners are heterozygous (HbAS) or “sickle cell trait”.
Rare forms of sickle-cell disease can be compound heterozygous; sickle-haemoglobin C disease (HbSC), sickle beta-plus-thalassaemia (HbS/β+) and sickle beta-zero-thalassaemia (HbS/β0).
In SCD; the body makes sickle-shaped red blood cells (RBCs are crescent shaped). Normally the RBCs are disc-shaped and move easily through the blood vessels, carrying oxygen from the lungs to the tissues. Sickle cells are sickle / crescent shaped which are stiff, sticky and tend to block blood flow in the blood vessels of the limbs and organs causing pain and organ damage.
Down’s Syndrome and Recurrence
Down Syndrome: Down syndrome (DS) or trisomy 21 is the most common genetic disorder with a prevalence of 1 in 660 live births

DS is of 3 types
Free trisomy 21 - Characterized by the presence of three complete copies of chromosome 21; occurring in about 90-95% of DS cases; > 90% of the cases of chromosomal meiotic nondisjunction are maternal in origin, about 5% due to an additional paternal extra chromosome and 2% is due to post-zygotic mitotic non-disjunction.
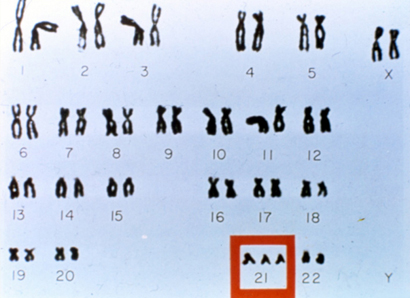
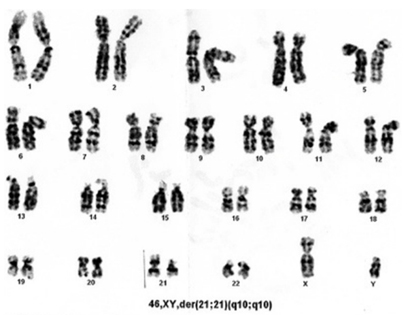
Translocations: Attribute to 1-7% of DS cases; Robertsonian translocation involving chromosomes 14 and 21 being the most common type.
Mosaicism - Characterized by 2 or more cell lines, some cells containing 46 chromosomes and others with 47 chromosomes (with an extra chromosome 1), is reported in 1-7% of DS cases.
DS phenotype is complex and variable
It present a combination of dysmorphic features and developmental delay. Intellectual disability is universal, muscular hypotonia (99%), upslanted palpebral fissures (90%), microcephaly (85%), flat occipital (80%), joint hyperextension (80%), broad hands with short fingers (70%), short stature (60%), clinodactyly of fifth finger (50%), epicanthal fold (40%), low-set ears (50%), single palmar crease (40%), atlantoaxial instability (15%) and on average, 50-70% have congenital heart defects, such as ventricular septal defect, atrial septal defect, tetralogy of Fallot, patent ductus arteriosus and atrioventricular septal defect.
For DS, a well-established risk factor is advanced maternal age at conception. The estimated risk for foetal trisomy 21 for 20 years women at 12 weeks of gestation is about 1/1000 & delivering an affected baby at term is 1/1500. The risk for this aneuploidy for a woman aged 35 years at 12 weeks of gestation is about 1/250 and delivering an affected baby at term is 1/350.
Risk of recurrence
Women < 35 years of age at previous trisomy 21, the revised risk is the age-related risk times 3.5.
Women ≥35 years of age at previous trisomy 21, the revised risk is the age-related risk times 1.7
Robertsonian translocations involving chromosome 13, 14, 15 or 22 and the chromosome 21, the recurrence risk is upto17% when the mother is the carrier and upto 1.4% father is the carrier of this balanced translocation.
If one of the parents is the carrier of a balanced translocation involving two chromosomes 21, the recurrence risk of DS is 100%
Individual with DS; theoretical risk to have a child with DS is 50% to 66%. However, Rate of fetal demise between 11 weeks and term is about 43% for trisomy 21; chances of birth of a child with DS decreases. Individuals with mosaicism, the maximum theoretical recurrence risk is as high as 50% (dependent upon the proportion of trisomic gonadal cells)
Serum screening
Elevated β-hCG (produced from placenta), elevated NH-A (corpus luteum and placenta) and low levels of AFP (produced from yolk sac and fetal liver) and uE3 (placenta); may be suggestive of the presence of DS fetus. The quadruple test has expected detection rate and false-positivity of 79-82% & 6.5-7.8%, respectively.
Ultrasound screening for DS
Genetic Sonogram
Objective is the detection of major and soft markers of aneuploidy. Increased nuchal translucency in the first trimester; Second trimester of gestation - lack of visualization of the nasal bone, reduced femur and humerus length, mild pyelectasis, hyperechoic bowel and echogenic intracardiac focus.
Prenatal invasive methods
Chorionic villus sampling (CVS)
Aspiration of trophoblastic tissue under continuous ultrasound guidance, performed via trans-abdominal route; done in first trimester of pregnancy (12-13 weeks of pregnancy). Risk miscarriage associated to this procedure is about 1/300.
Amniocentesis
Done at 16 weeks of gestation; a small sample of amniotic fluid aspirated transabdominally under ultrasound guidance. The procedure-related foetal loss rate is about 1/500
After obtaining foetal cells
Fluorescence in situ hybridization (FISH) has allowed the prenatal diagnosis of most frequent trisomies (21, 13, 18) and aneuploidy of sex chromosomes quickly and accurately, obtaining result from one to two days.
Polymerase chain reaction quantitative fluorescent (QF-PCR) are also be used for a rapid diagnosis of aneuploidies. Conventional karyotype analysis is the gold standard for the prenatal diagnosis of numerical and major structural chromosomal abnormalities; is labour intensive and requires an average reporting time of 14 days.
QF-PCR technique presents 95.4% sensitivity, 100% specificity, 99.5% efficiency for diagnosis of DS.
Importance of the prenatal diagnosis of DS is to provide the needed healthcare for the child, to prepare the family emotional and psychologically and to plan early intervention therapies.
Genetic causes of Down syndrome and associated recurrence risks
|
Nondisjunction Down syndrome |
Translocation Down syndrome |
Mosaic Down syndrome |
Frequency (% of cases) |
95% |
3–4% |
1–2% |
Cytogenetic findings |
An extra copy of chromosome 21 (free trisomy) as the result of meiotic nondisjunction (leading to 47 chromosomes) |
An unbalanced structural chromosome rearrangement involving chromosome 21 |
A mixture of cells that contain 46 chromosomes and cells that contain an extra copy of chromosome 21 (a total of 47 chromosomes) |
Etiology |
Maternal nondisjunction is causative in 90% of cases, and paternal nondisjunction is in the remaining 10% of cases. |
Robertsonian translocations between 21q and another acrocentric chromosome (13, 14, 15, 21 or 22) account for most familial cases. |
It may occur as the result of an early “trisomy rescue” or early somatic nondisjunction error. |
Recurrence risk |
For trisomy 21: |
If neither parent carries a balanced translocation, the Down syndrome recurrence risk is low, probably similar to that of nondisjunction trisomy 21. |
For a couple whose child has mosaic Down syndrome, the recurrence risk is similar to that of nondisjunction trisomy 21, although this may be an overestimate for some families. |
|
Maternal age <35 at previous trisomy 21, the revised risk is the age‐related risk times 3.5. |
Recurrence risk for translocation carriers is dependent on the type of translocation and the sex of the carrier parent. |
|
|
For any trisomy: |
|
|
|
Maternal age <35 at previous trisomy 21, the revised risk is the age‐related risk times 1.3. |
|
|
|
Maternal age ≥35 at previous trisomy 21, the revised risk is the age‐related risk times 1.5. |
|
|
Type of translocation |
Risk for unbalanced translocation at time of amniocentesis |
Other concerns |
rob(13q21q) |
10–17% if mother is carrier |
risk for translocation trisomy 13 |
|
<0.5% father is carrier |
|
rob(14q21q) |
15% if mother is carrier |
risk for uniparental disomy (UPD) 14 |
|
1.4% if father is carrier |
|
rob(15q21q) |
0–11% if mother is carrier |
risk for UPD 15 |
|
<0.5% if father is carrier |
|
rob(21q21q) |
100% if either parent is carrier |
____ |
rob(21q22q) |
13% if mother is carrier |
____ |
|
1.4% if father is carrier |
|
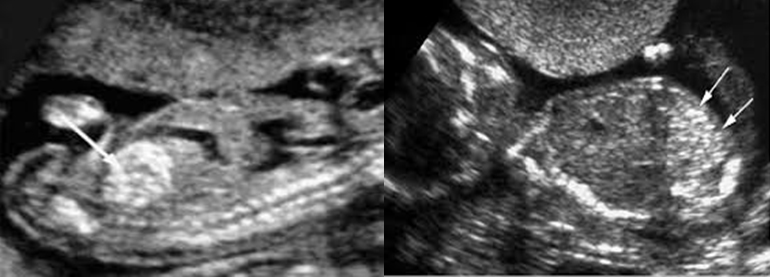
Hypoplastic Foetal Nasal Bones
Absent or Hypoplastic Nasal Bones
3 distinct lines
The first 2 lines; are horizontal and parallel to each other, resembling an ‘‘equal sign.’’ The top line represents the skin and the bottom line, which is thicker and more echogenic than the overlying skin, represents the nasal bone.
A third line, represents the tip of the nose.
When the nasal bone line appears as a thin line, less echogenic than the overlying skin, it suggests that the nasal bone is not yet ossified and is classified therefore as being absent.
In chromosomally normal foetus; Absent nasal bone is < 1% in white populations & 10% in Afro-Caribbean populations
Trisomy 21 is associated with abnormal flow in ductus venosus and maxillary hypoplasia.
NOT visible in I trimester or < 2.5 mm in the mid-sagittal section of the foetal profile in II trimester
Nasal bone was absent in 65 % of foetuses with trisomy 21 but only in 0.8 % of chromosomally normal foetuses
In the second trimester, this marker becomes less predictive with absent NB seen in 30–40 % of foetuses with trisomy 21 and 0.3–0.7 % of chromosomally normal foetuses
Nasal hypoplasia of the NB include an absolute cutoff of < 2.5 mm; gestational age-related cutoff of <2.5th or <5th percentile, a ratio of
BPD/NB length or multiples of the median for gestational age with <0.75 MoM being the cutoff for abnormal NB measurement
Considering NB hypoplasia or absent nasal bone as a single category, the finding was seen in 50–60 % of foetuses with trisomy 21 and 6–7 % of chromosomally normal foetuses
There is natural variation in the appearance of the NB.
Absence of the NB at or before 13 weeks gestation can be a result of delayed ossification instead of absence or hypoplasia.
Similarly, ethnic variations exist in the presence and size of the nasal bone
Increased Foetal Nuchal Translucency
Increased Foetal Nuchal Translucency thickness
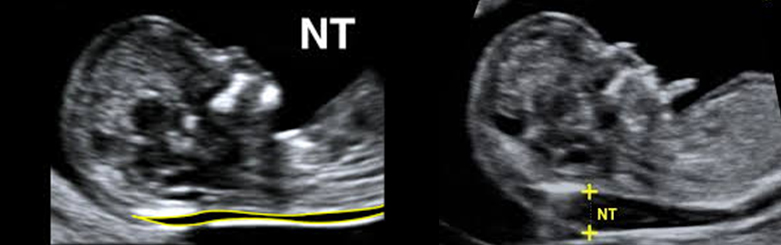
> 95th centile for CRL
maximum thickness of the subcutaneous translucency between the skin and soft tissue overlying the cervical spine
Sensitivity & false-positive rate of foetal NT in screening for trisomy 21 is similar in both singleton and DADC pregnancies
DAMC twins; increased NT (> 95th centile) at the 11–14-week scan – 4 fold increase in risk for TTTS syndrome
Increased NT in recipient foetus may be due to heart failure due to hypervolemic congestion but with advancing gestation; diuresis tends to correct the hypervolemia and reduce heart strain, resolving both congestive heart failure and increased NT.
1. 1/3 (28.7%) have chromosomal abnormalities; 75% of these are trisomy 21 or 18
2. Foetal cardiovascular, pulmonary defects, skeletal dysplasias, congenital infection, metabolic and hematologic disorders
3. Cardiac defects (abnormalities of heart and great arteries) 4.8 to 32.1%
4. Venous congestion in the head and neck,
a. Superior mediastinal compression (diaphragmatic hernia or narrow chest in skeletal dysplasia),
5. Altered composition of the extracellular matrix (altered gene dosage effects),
6. Abnormal or delayed development of the lymphatic system,
7. Foetal anemia or hypoproteinemia,
8. Congenital infection.
Approximately 30% of affected foetuses die between 12 weeks of gestation and term NO significant association between foetal NT & maternal serum free b-hCG or PAPP-A in either trisomy 21 or chromosomally normal pregnancies Trisomy 21 is associated with nasal hypoplasia, increased nuchal fold thickness, cardiac defects, intracardiac echogenic foci, duodenal atresia and echogenic bowel, mild hydronephrosis, shortening of the femur and humerus, sandal gap, and clinodactyly or mid-phalanx hypoplasia of the fifth finger.
In DADC twins; chance that at least 1 foetus is affected by a chromosomal defect is twice as high as in singleton pregnancies In monozygotic twins, the risk for chromosomal abnormalities is the same as in singleton pregnancies Relative proportion of DADC to DAMC twins is approximately 2-to-1; thus the prevalence of chromosomal abnormalities in twin pregnancies is expected to be approximately 1.6 times that in singleton pregnancies Increased NT is found in 4.4% of chromosomally normal foetuses, which are at a significant risk of foetal malformations, skeletal dysplasia, genetic syndromes and delayed brain development
Heart defects, ranging from 4.8 to 32.1%
0.8/1000 NT < 95th centile; 63.5/1000 NT > 99th centile; 56% NT > 95th centile Translucent area disappears after 14 weeks gestational age, when the subcutaneous tissue becomes more echogenic. NT is therefore a transient phenomenon
Hyperextension foetal neck can increase the NT measurement artificially by 0.6 mm, and flexion can decrease the measurement by 0.4 mm
Umbilical cord in neck in 5–10%; falsely increased NT; adding about 0.8 mm to the measurement; appropriate to use the smaller measurement.
NT |
Adverse outcome |
Born Healthy |
Miscarriage |
Major malformation |
Cardiac Abnormality |
95th centile and 3.4 mm |
5% |
|
8% |
2.5% |
2.4% |
3.5 to 4.4 mm |
30% |
70% |
|
|
7.1% |
4.5 to 5.4 mm |
50% |
50% |
|
|
12.3% |
5.5 to 6.4 mm |
80% |
30% |
|
|
16.7% |
> 6.5 mm |
|
15% |
80% |
45% |
35.6% |
Chromosomally normal foetuses; with NT > 95th centile and normal foetal morphological examination at 20 weeks; a favourable pregnancy outcome increases remarkably.
Caution is recommended when the NT is ≥ 6.5 mm.
Pathophysiology of Increased NT
In normal foetuses, collagen VI was only sparsely present in the dermis; which increased tremendously in trisomy-21 foetuses
Triplication of the genes encoding for collagen VI; the genes are located on chromosome 21 (COL6A1 and COL6A2).
Overexpression of genes of mRNA of the a1 chain of collagen VI which binds hyaluronan
Lymphatic and blood vessels are neither dilated nor hyperplastic
NT as an interstitial oedema, due to the presence of large amounts of hyaluronan
Overexpression of superoxide dismutase gene; also on chromosome 21; it stabilizes hyaluronan against free radicals.
Altered composition of the extracellular matrix - collagen metabolism (achondrogenesis type II), abnormalities FGFR (achondroplasia, thanatophoric dysplasia) or peroxisomal disorder (Zellweger syndrome).
Lymphatic abnormalities
Malformations of both the main jugular lymphatic trunk & peripheral lymphatic system; impaired lymphatic drainage; lack of connection with the venous system in these monosomy-X
Enlargement of these lymphatic sacs due to aortic arch malformations
Monosomy-X foetuses; peripheral lymphatic vessels are either absent or hypoplastic;
In trisomic and euploid foetuses with nuchal oedema these vessels were numerous and dilated
Using TVS (transvaginal ultrasound) in I -trimester foetuses with increased NT; enlarged lymphatic sacs could be visualized as Translucent cystic areas in foetal neck in the majority of cases, assumed to be the jugular lymphatic sacs
Enlargement of the jugular lymphatic sacs is due to delay in reconnection with the venous system or due to increased overall expression of the hyaluronic acid receptor
With continued development, the lymphatic sacs are remodelled into lymph nodes and are reconnected to the venous system where upon the excess fluid drains away, which explains the TRANSIENT nature of the increased NT.
At 14 weeks of gestation; drop in placental & peripheral resistance leads to draining of excess fluid.
In Turner's syndrome, increased NT - Abnormal development of the jugular lymphatic sacs.
Enlarged lymphatic jugular sacs become only partly re-organized and do not get proper reconnection into the jugular veins, leading to large amount of fluid in the posterior neck region, which is PERSISTENT & not transient and is called `hygroma colli'; delay in this process explains local accumulation of in the nuchal region and not in other areas of the foetal body
In normal embryos; main lymphatics develop from the venous walls & subsequently lose their connections with the veins to form a separate lymphatic system, except for jugulo-axillary sacs, which drain the lymph to the venous system.
Impaired foetal movements, foetal akinesia deformation sequence
Venous congestion
Congenital diaphragmatic hernia, oesophageal atresia causes mediastinal compression and increased impedance to venous return leading to venous congestion of the head and neck
In skeletal dysplasia; increased intrathoracic pressure occurs due to impaired thoracic growth
Foetal infection
First-trimester increased NT is NOT associated with foetal infection; in contrast to foetuses in II / III trimester hydrops.
Screen for maternal infections when increased translucency evolves into second- or third-trimester oedema or generalized hydrops.
Foetal hydrops at 12 weeks, due to a parvovirus infection, is due of cardiac failure is due to (a temporary) myocarditis rather than anaemia
Cardiac failure
Absent or reverse flow a wave DV
90% of chromosomally abnormal; in chromosomally normal foetuses with cardiac defects
Increased levels of atrial and brain natriuretic peptide mRNA in foetal hearts indicating heart strain
Triploidy
Additional chromosome complement - paternal (diandric) - partially molar placenta; b-hCG is greatly increased, PAPP-A is mildly decreased.
Maternal - Digynic triploidy - small normal-looking placenta and severe asymmetrical foetal growth restriction, markedly decreased maternal serum free b-hCG and PAPP-A.
COMBINED I trimester NT and PAPP-A with II trimester free b-hCG, estriol and inhibin A, claiming a potential sensitivity of 94% for a 5% false-positive rate.
In the mid-trimester scan minor foetal defects or markers are common and they are not usually associated with any handicap, unless there is an associated chromosomal abnormality.
Hyperechogenic bowel (intra-amniotic bleeding) and relative shortening of the femur (placental insufficiency) may well be related to serum biochemistry (high free b-hCG, high inhibin-A, low estriol - placental damage) and should be considered together in estimating the risk for trisomy 21.
Increased Nuchal fold thickness
A thickened NF- ≥ 6 mm at 15–20 weeks
Nuchal index is the mean nuchal fold/mean biparietal diameter (BPD) X 100. A value of 11 or greater has a sensitivity of 50% and a specificity of 96%
Noonan syndrome
Craniofacial abnormalities, brachicephaly, hypertelorism, low set ears, broad nose, full lips, cardiac anomalies (pulmonic stenosis, atrial septal defects, obstructive cardiomyopathy), hydrops foetalis and pleural effusion
If isolated:
a. Women with no prior screening, regardless of age - amniocentesis
b. Women with prior first, second-trimester screening - readjust the risk based on previous testing
c. Women with negative CVS, amniocentesis or NIPT for Down syndrome: no further assessment
d. Women with normal karyotype but a thickened NT that persisted as thickened NF into the second trimester: Noonan syndrome and specific diagnostic testing.
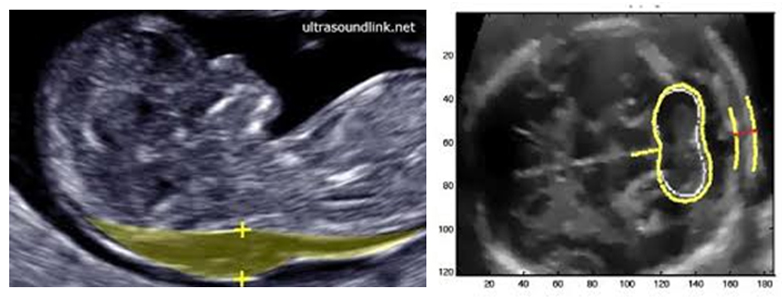
Pregnancy Pre-Eclampsia Screening – 12 weeks
I trimester & Pre-eclampsia
Preeclampsia is a major reason for maternal and perinatal morbidity and mortality; consequent of impaired placentation. Risk is higher when the disease is severe and of early onset requiring delivery before 34 weeks Challenge is for early identification & undertaking the measures to improve placentation and reduce the prevalence of the disease. Combination of maternal medical and obstetric history, uterine artery pulsatility index (PI), mean arterial pressure (MAP) and maternal serum pregnancy-associated plasma protein-A (PAPP-A) and placental growth factor (PlGF) at 11–13 weeks’ gestation.
Maternal age > 35 years, increasing weight, Afro-Caribbean / South Asian origin, previous pregnancy with PE, IVF conception and a medical history of chronic hypertension, pre-existing diabetes mellitus and systemic lupus erythematosus or antiphospholipid syndrome, increase the risk for development of PE.
Aetiology impaired placentation due to inadequate trophoblastic invasion of the maternal spiral arteries; reduced placental perfusion leading to placental ischaemia and release of inflammatory factors, platelet activation, endothelial dysfunction, maternal renal dysfunction or abnormal oxidative stress.
Pregnancy-associated plasma protein A (PAPPA) is a syncytiotrophoblast derived metalloproteinase; it enhances the mitogenic function of the insulin-like growth factors; important for placental growth and development significant relationship between PAPP-A levels < 5th centile and IUGR, preterm delivery, PE, and stillbirth
Combination of I Tri PAPP-A and II Tri sFlt-1/PlGF ratio (DR 87.5% ; FPR 5%)
Angiogenic Factors
Pro-Angiogenic Markers
Vascular Endothelial Growth Factor (VEGF) and Placental Growth Factor (PlGF)
Placental growth factor (PIGF)
It is a glycosylated dimeric glycoprotein; member of vascular endothelial growth factor sub-family. PlGF has both vasculogenetic and angiogenetic functions; controlling the expansion of the capillary network.
Curvilinear relationship with gestational age; increase in I/II trimesters; peak at 30 weeks & subsequently decrease
Maternal serum levels of PlGF at 11–13 weeks’ gestation are decreased in pregnancies with foetal aneuploidies and those with impaired placentation, resulting in PE and delivery of small-for-gestational-age neonates.
First-trimester maternal serum concentrations of PAPP-A and PlGF Mom are affected by gestational age at screening, maternal weight, racial origin, cigarette smoking, conception by IVF, nulliparity and pre-existing diabetes mellitus.
Serum PlGF is also affected by maternal age
• PlGF decreases with gestational age and maternal weight
• It is higher in Afro-Caribbean and South Asian race than in Caucasians
• Higher in parous than nulliparous women
• Higher in smokers than non-smokers.
• PAPP-A & PlGF MoM are significantly reduced at 11–13 weeks’ gestation in women who subsequently develop PE.
Anti-Angiogenic Markers
Serum sFlt-1
Circulates freely in the serum; binds and neutralizes VEGF and PlGF
Excessive placental production of sFlt-1 (antagonist of VEGF and PlGF) contributes to the pathogenesis of PE
Defective early placentation with impaired trophoblast invasion and restricted remodeling; early-onset PE, resulting in reduced uteroplacental perfusion.
II/III trimester sFlt-1/PlGF ratios - detection rate of 87.5% , FPR 10% for early PE in a low-risk population
• It increases with gestational age and maternal age
• decreases with maternal weight
• Increased Afro-Caribbean race
• Increased in pregnancies conceived by IVF
• Lower in parous than nulliparous women
• Pregnancies complicated by PE; have decreased serum PlGF MoM; increased sFlt-1 MoM.
Soluble Endoglin (sEng)
Truncated form of receptor for transforming growth factor (TGF)-β1 and TGF-β2; anti-angiogenic factor; interferes with production of nitric oxide, vasodilation, and capillary formation.
sEng along with sFlt-1 to induce a severe PE-like disease
Inhibin-A and Activin-A
Members of TGF-β family; Inhibin-A - negative feedback of gonadotropins
Concentrations of inhibin-A and activin-A; are increased in women who will have PE
Inhibin-A and activin-A have been shown to be increased prior to 14 weeks in PE pregnancies
Fetal Hemoglobin
Upregulation of HbF genes; accumulation of extracellular HbF in the vascular lumen in PE placentas
With unexplained placental hypoxia - upregulation of placental HbF genes and proteins - induce formation of ROS, oxidative damage and leakage of the feto-maternal barrier in PE.
Leak of feto-maternal barrier results in endothelial dysfunction, hypertension, and proteinuria.
Significantly elevated levels of HbF and antioxidant alpha(1)-microglobulin (A1M) in the PE group
Cell-Free DNA
Human fetal DNA triggers in vitro activation of NF-κB, with resultant increased IL-6 production in both pregnant and nonpregnant donors
There is graded response between the quantity of fetal DNA and the risk of developing PE; highest levels HELLP syndrome. Increase in shed DNA in PE reflects the increased hypoxic cell death
Foetal Echogenic Bowel
Seen in 0.4% to 1.8% of normal foetuses, resolves spontaneously in 19.7% of cases
Chromosomal (Down syndrome), Cystic fibrosis, Congenital intrauterine infections (cytomegalovirus, parvovirus, rubella, varicella and toxoplasmosis), Intrauterine growth restriction (IUGR), Intrauterine foetal demise (IUFD).
Risk of IUGR is increases with raised MSAFP
Relative risk - 1.6 for IUGR & 8.6 for intrauterine foetal demise
Dilated hyperechogenic bowel - higher risk for gastrointestinal bowel obstruction and meconium peritonitis
Swallowing of amniotic fluid contaminated by intraamniotic blood
IUGR: 9.9–23%, IUFD: 7.3–10%, cystic fibrosis: 2.3–7.6% and congenital infection: 1–10%
Incidence of aneuploidy in foetuses with isolated hyperechogenic bowel is 3.3 to 16% (Trisomy 21)
Foetal Echogenic Bowel
Pyelectasis; dilatation exclusively involving the foetal renal pelvis, is a common ultrasonographic finding
Prevalence among normal foetuses - 0.6 - 4.5%
Isolated pyelectasis is seen in 0.7% of foetuses at 16 to 26 weeks gestation; 2% in Down syndrome
Mild renal pyelectasis has an increased risk of aneuploidy, particularly trisomy 21 when another risk factor such as advanced maternal age (≥ 36 years) or
associated anomalies are involved
Renal pelvic anteroposterior diameter is ≥ 4mm in < 20 weeks of gestation; ≥ 7 mm in < 28 weeks of gestation and ≥ 10 mm at any gestational age.
In Euploid foetuses
Mild renal pyelectasis resolves antenatally in 80% of cases; postnatally in 17% and 3% of the foetuses might require postnatal work up.
From Grade III upwards, when the renal pelvis along with both major and minor calyceal dilation; postnatal referral and examination of the infant by paediatric urologist is warranted to minimize renal injury and scarring.
Foetal Echogenic Bowel
Bone length < 5th centile for gestational age, observed in 0.4 to 3.9% of normal foetus
Foetuses of Asian descent - shorter mean FL & foetuses of African American descent - longer mean FL when compared to foetuses from Caucasian mothers.
Shortening for HL is more sensitive and specific than FL
Associated deformity, demineralization, bowing or fracture of the long bones to be looked for
Early sign of placental dysfunction and warrant increased antenatal surveillance for growth assessment
Short long bones may be observed in skeletal dysplasias, genetic syndromes, fetuses with growth restriction, chromosomal
abnormalities and also in constitutionally small babies.
In majority; Serial growth scans, Uterine artery Doppler screening, foetal karyotyping, genetic testing may be performed to differentiate constitutionally small babies from those with skeletal dysplasias and chromosomally abnormal babies.
This helps in counselling regarding the exact nature; likelihood course of the disease; prognosis and also assess the recurrence risks.
